Streamline Clinical Trials
A turnkey logistics solution for stress-free clinical trials
Streamline Clinical Trials when timelines shift, sites change, and temperature requirements stay strict. CRYOPDP provides a single, coordinated clinical trial logistics solution with documented processes, compliance controls, and defined escalation procedures when conditions change.
Early involvement supports decision-making on setup, including packaging strategy, lane planning, documentation, and site and delivery requirements. One point of operational ownership keeps decisions clear and execution consistent across shipments and sites.
What do we offer?
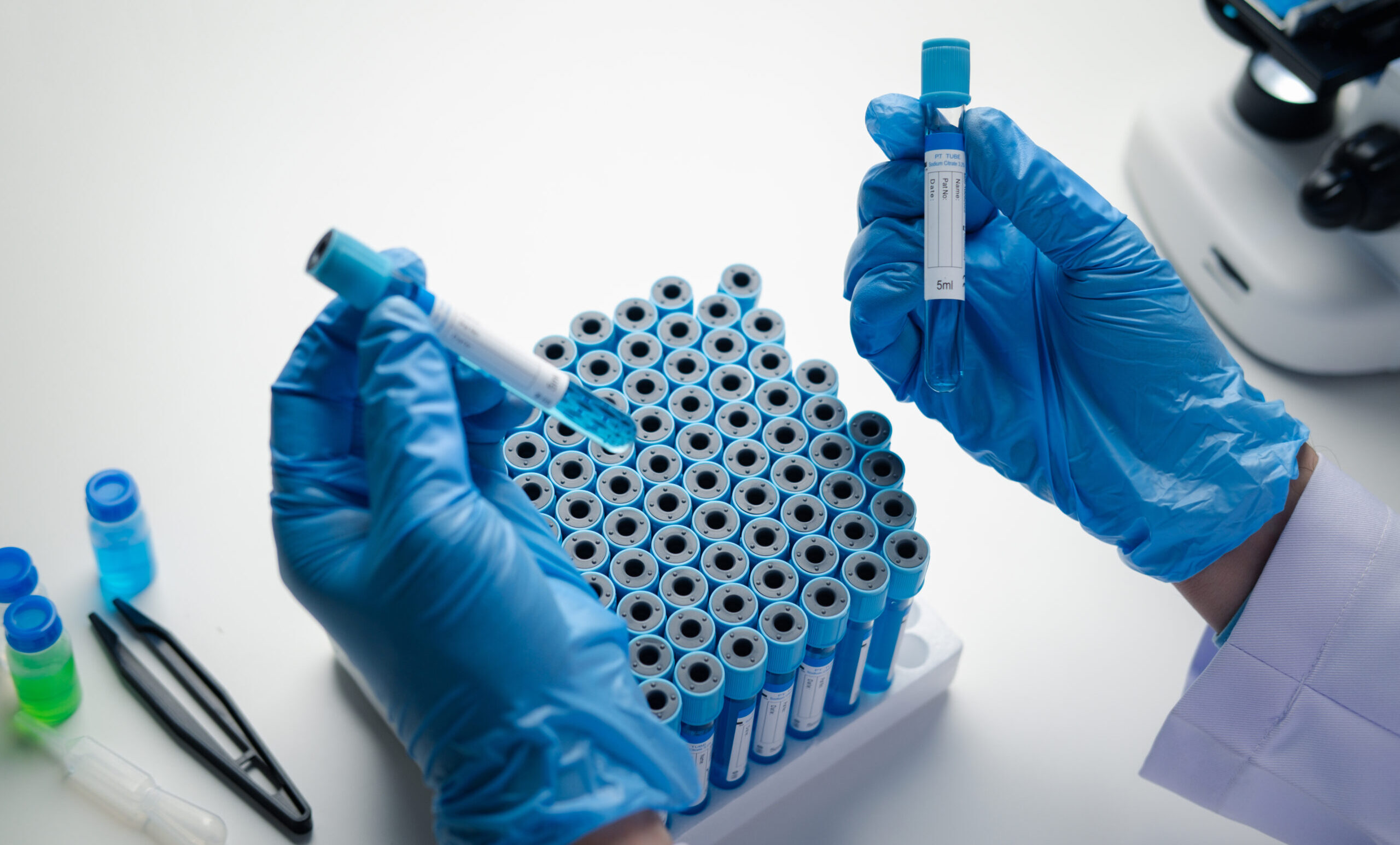
A single operational set-up across the clinical trial lifecycle, from pre-trial to post-trial activities.
Services include pre-trial consultancy, temperature-controlled logistics for biological samples and investigational medicines during the trial, shipment status and data reporting, and post-trial laboratory sample destruction where required.
Pre-Trial
During protocol design, CRYOPDP advises on clinical trial kit configuration and primary temperature-controlled packaging, manages ordering from selected suppliers, and prepares ready-to-use kits. Services include clinical label design and production, kit assembly, and distribution planning for delivery to investigational sites. Guidance also covers temperature-controlled shipment requirements for investigational medicines and biological samples. For international trial planning standards, refer to the ICH Clinical Guidelines.
During Trial
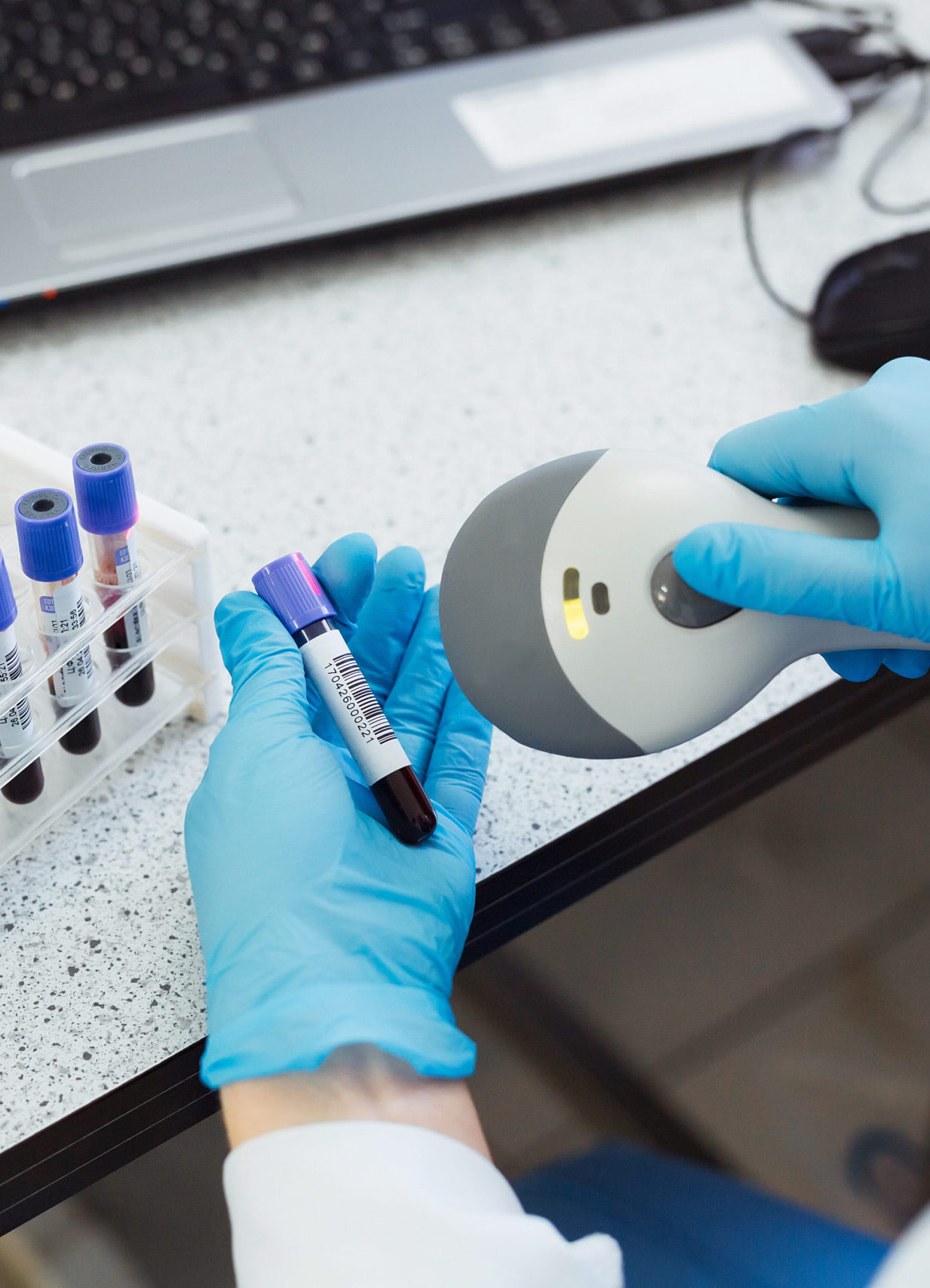
Temperature-controlled logistics are managed across participating sites, with stock monitoring, expiry tracking, and planned replenishment of kits and labels based on site demand and visit schedules.
Post-Trial
Post-trial activities include destruction of stored biological samples and expired kits where required, plus kitting and logistics data reporting to support compliance documentation.
A turnkey partner to streamline your clinical trials
-

Advise
on tubes and primary temperature-controlled packaging -

Design
and production of clinical sample labels -
Ordering
from selected suppliers -

Assembly
of clinical trial sample kits -

Transport and Logistics
of ready-to-use kits to locations across Europe -

Temperature-controlled
transport of biological samples and investigational medicines -

Stock management and expiry control
for clinical trial sample kits -
Destruction
of expired kits